Grupo de Acuicultura y Biodiversidad (GAB-UPV)
Instituto de Ciencia y Tecnología Animal
Universitat Politècnica de València
Contact
Dr. Juan F. Asturiano
jfastu@dca.upv.es
Phone: +34 96 387 93 85
Address
Instituto de Ciencia y Tecnología Animal (Edificio 7G)
Universitat Politècnica de València
Camino de Vera s/n
46022 Valencia (Spain)
Group description
The Grupo de Acuicultura y Biodiversidad (GAB-UPV) is part of the Instituto de Ciencia y Tecnología Animal at the Universitat Politècnica de València (www.upv.es). The group is formed by 5 professors, 2 postdocs, 1 technician and several Ph D and Master students. Part of the members work on nutrition aspects while the rest is dedicated to several aspects of fish reproduction (see main lines of research).
The professors teach several subjects (Aquaculture, Hydrobiology, Zoology, Biology) on several degrees and as part of the Máster Oficial Interuniversitario en Acuicultura (Reproduction, Nutrition and feeding, Design and management of facilities, Aquaculture engineering) with 110 students during the last 5 years, and being part of the Doctorate Program of this University, titled Animal Production Science and Technology (Animal Science Department).
Main lines of research
-Control of the European eel reproduction:
Hormonal treatments for the sexual maturation and spawning. Physiology of the gonad development. Endocrine control. Key-gene expression. Effect of the environmental parameters. Techniques for the evaluation and cryopreservation of gametes. Reproduction in captivity.
-Gametogenesis in Teleostean females:
Oocyte development. Vitellogenesis. Maturation. Ovulation. Artificial fertilization. Quality of eggs and spawnings. Histological techniques. Endocrine control and induced maturation.
Links
– Blog “Acuicultura, Fisiología y Reproducción”: informal description of group activities, projects, publications, etc. www.acuiculturaenvalencia.blogspot.com.es
– Instituto de Ciencia y Tecnología Animal (ICTA): http://www.icta.upv.es
– Master Interuniversitario de Acuicultura: http://macuicultura.webs.upv.es
– Curso On-line de Acuicultura: http://www.cursodeacuicultura.upv.es
– PRO-EEL Project. Reproduction of European Eel: Towards a Self-sustained Aquaculture. http://www.pro-eel.eu
List of publications
1. V. Gallego, M.C. Vílchez, D.S. Peñaranda, L. Pérez, M.P. Herráez, J.F. Asturiano, F. Martínez-Pastor. The subpopulation pattern of eel sperm is affected by post-activation time, hormonal treatment and thermal regime. Reproduction, Fertility and Development, in press.
2. V. Gallego; F. MArtínez-Pastor; I. Mazzeo; D.S. Peñaranda; M.P. Herráez; J.F. Asturiano; L. Pérez. Intracellular changes in Ca2+, K+ and pH after sperm motility activation in the European eel (Anguilla anguilla): Preliminary results. Aquaculture 418-419: 155-158. 2014.
3. D.S. Peñaranda; I. Mazzeo; J. Hildahl; V. Gallego; R. Nourizadeh-Lillabadi; L. Pérez; J.F. Asturiano; F.A. Weltzien. Molecular characterization of three GnRH receptor paralogs in the European eel, Anguilla anguilla: Tissue-distribution and changes in transcript abundance during artificially induced sexual development. Molecular and Cellular Endocrinology 369(1-2): 1-14. 2013.
4. V. Gallego; P.C.F. Carneiro; I. Mazzeo; M.C. Vílchez; D.S. Peñaranda; C. Soler; L. Pérez; J.F. Asturiano. Standardization of European eel (Anguilla anguilla) sperm motility evaluation by CASA software. Theriogenology 79: 1034-1040. 2013.
5. V. Gallego; L. Pérez; J.F. Asturiano; M. Yoshida. Study of puffer fish (Takifugu niphobles) sperm: development of methods for short-term storage, effects of different activation media and role of intracellular changes in Ca2+ and K+ in the initiation of sperm motility. Aquaculture 414-415: 82-91. 2013.
6. V. Gallego, L. Pérez, J.F. Asturiano; M. Yoshida. Relationship between spermatozoa motility parameters, sperm/egg ratio, and fertilization and hatching rates in pufferfish (Takifugu niphobles). Aquaculture 416-417: 238-243. 2013.
7. S.R. Sørensen; V. Gallego; L. Pérez; I.A.E. Butts; J. Tomkiewicz; J.F. Asturiano. Evaluation of methods to determine sperm density for the European eel, Anguilla anguilla. Reproduction in Domestic Animals 48(6): 936-944. 2013.
8. V. Gallego; D.S. Peñaranda; F. Marco-Jiménez; I. Mazzeo; L. Pérez; J.F. AsturianoComparison of two techniques for the morphometry study on gilthead seabream (Sparus aurata) spermatozoa and evaluation of changes induced by cryopreservation. Theriogenology 77: 1078-1087. 2012.
9. V. Gallego; I. Mazzeo; M.C. Vílchez; D.S. Peñaranda; P.C.F. Carneiro; L. Pérez; J.F. Asturiano. Study of the effects of thermal regime and alternative hormonal treatments on the reproductive performance of European eel males (Anguilla anguilla) during induced sexual maturation. Aquaculture 354-355: 7-16. 2012.
10. I. Mazzeo; D.S. Peñaranda; V. Gallego; J. Hildahl; R. Nourizadeh-Lillabadi; J.F. Asturiano; L. Pérez; F.A. Weltzien. Variations in the gene expression of zona pellucida proteins, zpb and zpc, in female European eel (Anguilla anguilla) during induced sexual maturation. General and Comparative Endocrinology 178(2): 338-346. 2012.
11. Á. Horváth; J.F. Asturiano; H. Rosenthal. On the biology of fish gametes: summary and recommendations of the Third International Workshop, Budapest and Gödöllo, Hungary, 2011. Journal of Applied Ichthyology 28(6): 863-864. 2012.
12. L. Pérez; D.S. Peñaranda; S. Dufour; S. Baloche; A.P. Palstra; G.E.E.J.M. van den Thillart; J.F. Asturiano. Influence of temperature regime on endocrine parameters and vitellogenesis during experimental maturation of European eel (Anguilla anguilla) females. General and Comparative Endocrinology 174: 51-59. 2011. doi: 10.1016/ygcen.2011.08.009.
13. D.S. Peñaranda; L. Pérez; V. Gallego; M. Jover; S. Baloche; S. Dufour; J.F. Asturiano. Molecular and physiological study of the artificial maturation process in the European eel males: from brain to testis. General and Comparative Endocrinology 166: 160-171. 2010.
14. D.S. Peñaranda; L. Pérez; V. Gallego; R. Barrera; M. Jover; J.F. Asturiano. European eel sperm diluent for short-term storage. Reproduction in Domestic Animals 45: 407-415. 2010.
15. H. Rosenthal; J.F. Asturiano; O. Linhart; A. Horvath. On the biology of fish gametes: summary and recommendations of the Second International Workshop, Valencia, Spain, 2009. Journal of Applied Ichthyology 26: 621-622. 2010.
Institute of Marine Science of Andalusia-ICMAN
Spanish National Research Council- CSIC
Contact
Elsa Cabrita
Elsa.cabrita@icman.csic.es
+34.956832612 ext 339
Address
ICMAN.CSIC
Av. República Saharaui 2
11510 Puerto Real Cádiz
Spain
Facilities Description
The center offers several wet laboratories and fish facilities for larval, juvenile and broodstock breeding. Research facilities allow the work in gene expression patterns, in situ and immune detection, histology, cryopreservation techniques and other several techniques. Researchers have expertise in Senegalese sole (S. senegalensis), gilthead seabream (S. aurata), European seabass (D. Labrax), zebrafish (D. rerio), mullet (C. labrosus) and toadfish (H. didactylus). Students are welcome for short stays to work in these areas and species.
Main research lines
Gametogenesis and fish reproduction
Larval rearing and cellular biomarkers for larval quality
Endocrine disruptors and xenobiotcs
Gamete quality and cryopreservation
Germ cell biotechnology
Relevant publications
1. Pacchiarini T, Olague E, Sarasquete C, Cabrita E. 2014. Busulfan administration produces sublethal effects on somatic tissues and inhibits gametogenesis in Senegalese sole juveniles. Histology and Histopathology (in press).
2. Pacchiarini T, Sarasquete C, Cabrita E. 2014. Development of interspecies testicular germ cells transplantation in flatfish. Reproduction, Fertility and Development (in press).
3. Pacchiarini T, Cross I, Leite R, Gavaia P, Ortiz-Delgado JB, Pousão-Ferreira P, Rebordinos L, Sarasquete C, Cabrita E. 2013. The Solea senegalensis vasa transcripts: molecular characterization, tissue distribution and developmental expression profiles. Reproduction, Fertility and Development 25,646-660.
4. Martínez-Páramo S, Diogo P, Dinis MT, Soares F, Sarasquete C, Cabrita E. 2013. Effect of two sulfur-containing amino acids, taurine and hypotaurine in European sea bass (Dicentrarchus labrax) sperm cryopreservation. Cryobiology 66 (3), 333-338.
5. Migaud, H., Bell G., Cabrita E., McAndrew B., Davie A., Bobe J., Herráez M.P., Carrillo M. 2013. Broodstock management and gamete quality in temperate fish. Reviews in Aquaculture 5 (1), S194-S223.
6. Martínez-Páramo S, Diogo P, Beirão J, Dinis MT, Cabrita E. 2012. Sperm lipid peroxidation explains differences in sperm quality during reproductive season in precocious European seabass (Dicentrarchus labrax) males. Aquaculture 358, 246-252.
doi: 10.1016/j.aquaculture.2012.06.010.
7. Fernández-Díez C, Pérez-Sanchiz R, Sarasquete C, Cabrita E, Herráez MP. 2012. New tools for genome preservation: grafting spermatogonia in brown trout (Salmo trutta). Journal of Applied Ichthyology 28, 916–918. doi: 10.1111/jai.12077.
8. Martínez-Páramo S, Diogo P, Dinis MT, Herráez MP, Sarasquete C, Cabrita E. 2012. Incorporation of ascorbic acid and α-tocopherol to the extender media to enhance antioxidant system of cryopreserved seabass sperm. Theriogenology 77, 1129-1136.
9. Yúfera, M., Halm, S., Beltran, S., Fusté, B., Planas, J.V., Martínez-Rodríguez, G. 2012. Transcriptomic characterization of the larval stage in gilthead seabream (Sparus aurata) by 454 pyrosequencing. Marine Biotechnology, 14: 423–435 – Enlace
10. Ortiz-Delgado, J.B., Iglesias, J., Sánchez, F.J., Cal, R., Lago, M.J., Otero, J.J., Sarasquete, C. 2012. A morphohistological and histochemical study of hatchery-reared European hake, Merluccius merluccius (Linnaeus, 1758), during the lecitho-exotrophic larval phase. Scientia Marina 76(2): 259-271
11. Beirão J, Zilli L, Vilella S, Cabrita E, Schiavone R, Herráez MP. 2012. Improving sperm cryopreservation with antifreeze proteins: effect on seabream plasma membrane lipids. Biology of Reproduction 86 (2) 59, 1-9.
12. Cabrita E, Ma S, Diogo P, Martínez-Páramo S, Sarasquete C, Dinis MT. 2011. The influence of certain aminoacids and vitamins in post-thaw fish sperm motility, viability and DNA fragmentation. Animal Reproduction Science 125, 189-195.
13. Beirão J, Soares F, Herráez MP, Dinis MT, Cabrita E. 2011. Changes in Solea senegalensis sperm quality throughout the year. Animal Reproduction Science 126, 122-129.
14. Cabrita E, Soares F, Beirão J, García-López A, Martínez-Rodríguez G, Dinis MT. 2011. Endocrine and milt response of Senegalese sole, Solea senegalensis, males maintained in captivity. Theriogenology 75, 1-9.
15. Cabrita, E., Sarasquete, C., S. Martínez-Páramo, V. Robles, J. Beirão, S. Pérez-Cerezales, M.P. Herráez. 2010. Cryopreservation of fish sperm: applications and perspectives. Journal of Applied Ichthyology 26, 623-635.
Investigación en Tecnologías de la Reproducción en Peces (ITRA-P-ULE)
Dpto Biología Molecular e Instituto de Desarrollo Ganadero
Universidad de León
Contact
Dr. Mª Paz Herráez
paz.herraez@unileon.es
Phone: +34987291912
Dr. Vanesa Robles
v.robles@unileon.es
Phone: +349871000-5678
Address
Dpt Molecular Biology, Cell Biology Area
University of León
24071, León (Spain)
Group description
Our group makes part of the group of Research on Biology of Reproduction and Related Biotechnologies in the University of León (ITRA-ULE). This group is recognized as “Group of Excellence” by Junta de Castilla y León. The fish division started their activities 15 years ago, focusing on sperm quality, and gametes/embryos cryopreservation in aquaculture. Our interests have evolved to a wider scope of subjects, including the study of model species. During this time we have formed several researches, published a high number of scientific papers, edited the book “Methods in reproductive aquaculture” and transferred our results to fish farming companies. At present the group is formed by 2 senior researchers 4 young researchers and 5 students. The professors teach several subjects related to Cell Biology, Histology and Organography in 3 different Degrees (Biology, Biotechnology and Environmental Sciences) as well as more specific subjects in Master programs (Master in Research Methods in Fundamental Biology and Biomedicine, and Master in Veterinary Sciences) and in Doctorate programs.
Main lines of research
1.- Fish spermatology: sperm quality and cryopreservation
2.- Cryobanking in fish: sperm, embryos and germinal cells
3- PGCs visualization, selection, cryopreservation and grafting
4- DNA damage in PGCs, spermatozoa and embryos
5- Epigenetic modifications in fish PGCs, spermatozoa and embryos
6- Gene expression in gametes and embryos
Species
1-Zebrafish (Danio rerio)
2- Trout (O. mykiss and S.trutta)
3- Sea bream (Sparus aurata)
Links
Web site of ITRA-ULE: http://itra.unileon.es/people/
University of León: www.unileon.es
Relevant publications
1. Cabrita, E.; Robles, V.; Alvarez, R.; Herráez, M.P. Cryopreservation of rainbow trout sperm in large volume straws: application to large scale fertilization. Aquaculture, 201 (3-4): 301-314 (2001).
2. E. Cabrita, V. Robles, L. Rebordinos, C. Sarasquete and M.P. Herráez. Evaluation of DNA damage in rainbow trout (Oncorhynchus mykiss) and gilthead sea bream (Sparus aurata) cryopreserved sperm. Cryobiology, 50(2): 144-153. (2005)
3. Cabrita, E., Robles, V. Herráez, MP. Editors of: Methods in Reproductive Aquaculture: Marine and Freshwater Species. Biology Series, CRCPress, Boca Raton, FL, USA 549pp. (2008).
4. S. Martínez-Páramo, S. Pérez-Cerezales, F. Gómez-Romano, G. Blanco, J.A. Sánchez, M.P. Herráez. Cryobanking as tool for conservation of biodiversity: Effect of brown trout sperm cryopreservation on the male genetic potential. Theriogenology, 71(4): 594-604. (2009)
5. S. Pérez-Cerezales, S. Martínez-Páramo, J. Beirão and M.P. Herráez. Fertilization capacity with rainbow trout DNA damaged sperm and embryo developmental success. Reproduction 139: 989-997 (2010).
6. S. Pérez-Cerezales, A. Gutiérrez-Adán, S. Martínez-Páramo, J. Beirão, MP. Herráez. Altered gene transcription and telomere length in trout embryo and larvae obtained with DNA cryodamaged sperm. Theriogenology. 76: 1234-1245. (2011).
7. V.Robles, M. Martí, J.C. Izpisúa-Belmonte. Study of pluripotency markers in zebrafish embryos and transient ES cell cultures. Zebrafish v8 (2011).
8. Beirão, J., Zilli, L., Vilella, S., Cabrita, E., Schiavone, R., Herráez, MP. Improving sperm cryopreservation with antifreeze proteins: effect on gilthead seabream (Sparus aurata) plasma membrane lipids. Biology of Reproduction. 86: 59, 1-9 (2012).
9. MF Riesco, F. Martínez Pastor, O. Chereguini, V.Robles. Evaluation of zebrafish (Danio rerio) PGCs viability and DNA damage using different cryopreservation protocols: Theriogenology 77 122-130 (2012).
10. Martínez-Pastor, F. and Robles V. Flow cytometric methods for sperm assessment. Spermatogenesis and Spermiogenesis: Methods and Protocols. Carrell DT and Aston K.I. (Eds), Methods Mol Biol. 927:175-86. (2013).
11. Cartón-García, F., Riesco M.F., Cabrita E., Herráez MP, Robles, V. Quantification of lesions in nuclear and mitochondrial genes of Sparus aurata cryopreserved sperm. Aquaculture 402-403: 106-112 (2013)
12. Labbe, C., Robles, V., Herráez, MP. Cryopreservation of gametes for aquaculture and alternative cell sources. En: Advances in Aquaculture Hatchery Technology. Burnell, G. (ed.), Woodhead Publishing pp 76-116 (2013).
13. Guerra SM, García Valcarce D., Cabrita E and Robles V. Analysis of transcripts in gilthead seabream sperm and zebrafish testicular cells: mRNA profile as a predictor of gamete quality. Aquaculture 406-407 (2013) 28-33 (2013).
14. Riesco MF. and Robles V. Cryopreservation causes genetic and epigenetic changes in zebrafish genital ridges PloS ONE 8(6): (2013).
15. Fernández-Díez, C, González-Rojo, S. Montfort, J. Le Cam, A. Bobe, J. Robles, V. Pérez-Cerezales, S and Herráez, M.P Inhibition of zygotic DNA repair: transcriptome analysis of the offspring in trout (Onchorynchus mykiss). Biology of Reproduction (In press).
Group of Comparative Molecular Physiology
Institute of Research and Technology in Food and Agriculture (IRTA)
Contact
Dr. Joan Cerdà
joan.cerda@irta.cat
Phone: +34 932309531
Address
IRTA-Institute of Marine Sciences (ICM, CSIC)
Passeig marítim 37-49
08003-Barcelona
Spain
Group description
The group is part of the Aquaculture Program of IRTA (http://www.irta.cat) at the Institute of Marine Sciences (ICM, CSIC; http://www.icm.csic.es) of Barcelona. The group is currently formed by the one group leader (J. Cerdà), one postdoc, and two PhD students.
Research topics
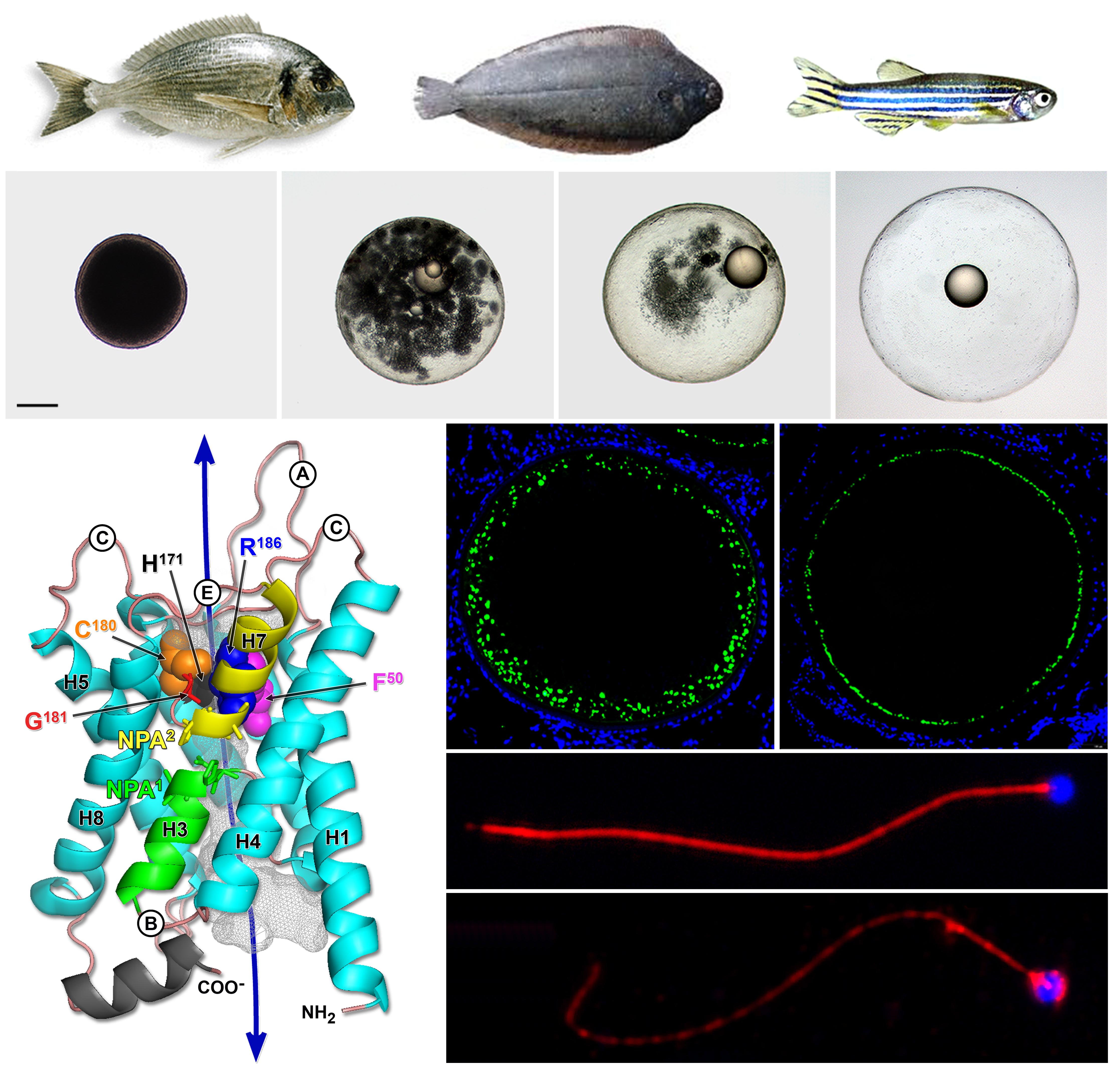
Our research interests deal with the molecular physiology and genomics of fish reproductive biology towards the development of new biotechnological applications in aquaculture, particularly for the control of reproduction and the long-term preservation of gametes, and the genetic improvement of egg quality. Current studies are focused on: (1) Molecular physiology of fish aquaporin water channels in health and disease; (2) Role aquaporin-mediated fluid homeostasis during gametogenesis and early embryogenesis; (3) Applications of solute-permeable aquaporins for the cryopreservation of gametes and embryos; and (4) Molecular endocrinology and genomics of flatfish gametogenesis.
Relevant publications
1. Fabra M., Raldúa D., Power D.M., Deen P.M.T., Cerdà J. (2005) Marine fish egg hydration is aquaporin-mediated. Science 307:545.
2. Agulleiro M.J., Anguis V., Cañavate J.P., Martínez-Rodríguez G., Mylonas C.C., Cerdà J. (2006) Induction of spawning of captive-reared Senegal sole (Solea senegalensis) using different delivery systems for gonadotropin-releasing hormone agonist. Aquaculture 257:511-524.
3. Fabra M., Raldúa D., Bozzo M.G., Deen P.M.T., Lubzens E., Cerdà J. (2006) Yolk proteolysis and aquaporin-1o play essential roles to regulate fish oocyte hydration during meiosis resumption. Developmental Biology 295:250-262.
4. Cerdà J., Chauvigné F., Agulleiro M.J., Marin E., Halm S., Martínez-Rodríguez G., Prat F. (2008) Molecular cloning of Senegalese sole (Solea senegalensis) follicle-stimulating hormone and luteinizing hormone subunits and expression pattern during spermatogenesis. General and Comparative Endocrinology 156:470-481.
5. Cerdà J., Mercadé J., Lozano J.J., Manchado M., Tingaud-Sequeira A., Astola A., Infante C., Halm S., Viñas J., Castellana B., Asensio E., Cañavate P., Martínez-Rodríguez G., Piferrer F., Planas J.V., Prat F., Yúfera M., Durany O., Subirada F., Rosell E., Maes T. (2008) Genomic resources for a commercial flatfish, the Senegalese sole (Solea senegalensis): EST sequencing, oligo microarray design, and development of the bioinformatic platform Soleamold. BMC Genomics 9:508.
6. Tingaud-Sequeira A., Chauvigné F., Lozano J., Agulleiro M.J., Asensio E., Cerdà J. (2009) New insights into molecular pathways associated with flatfish ovarian development and atresia revealed by transcriptional analysis. BMC Genomics 10:434.
7. Zilli L., Schiavone R, Chauvigné F., Cerdà J., Storelli C., Vilella S. (2009) Evidence for the involvement of aquaporins during the activation of sperm motility in the teleost gilthead seabream (Sparus aurata). Biology of Reproduction 81:880-888.
8. Lubzens E., Young G., Bobe J., Cerdà J. (2010) Oogenesis in teleosts: how fish eggs are formed. General and Comparative Endocrinology 165:367-389.
9. Chauvigné F., Tingaud-Sequeira A., Agulleiro M.J., Calusinska M., Gómez A., Finn R.N., Cerdà J. (2010) Functional and evolutionary analysis of flatfish gonadotropin receptors reveals cladal- and lineage-level divergence of the teleost glycoprotein receptor family. Biology of Reproduction 82:1088-1102.
10. Cerdà J., Douglas S., Reith M. (2010) Genomic resources for flatfish research and their applications. Journal of Fish Biology 77:1045-1070.
11. Chauvigné F., Lubzens E., Cerdà J. (2011) Design and characterization of genetically engineered zebrafish aquaporin-3 mutants highly permeable to the cryoprotectant ethylene glycol. BMC Biotechnology 11:34.
12. Zapater C., Chauvigné F., Norberg B., Finn R.N., Cerdà J. (2011) Dual neofunctionalization of a rapidly evolving aquaporin-1 paralog resulted in constrained and relaxed traits controlling channel function during meiosis resumption in teleosts. Molecular Biology and Evolution 28:3151-3169.
13. Chauvigné F., Verdura S., Mazón M.J., Duncan N., Zanuy S., Gómez A., Cerdà J. (2012) Follicle-stimulating hormone and luteinizing hormone mediate the androgenic pathway in Leydig cells of an evolutionary advanced teleost. Biology of Reproduction 87(2):35, 1-11.
14. Chauvigné F., Boj M., Vilella S., Finn R.N., Cerdà J. (2013) Subcellular localization of selectively permeable aquaporins in the male germ line of a marine teleost reveals spatial redistribution in activated spermatozoa. Biology of Reproduction 89(2):37, 1-17.
15. Chauvigné F., Zapater C., Gasol J.M., Cerdà J. (2014) Germ line activation of the luteinizing hormone receptor directly drives spermiogenesis in a non-mammalian vertebrate. Proceedings of the National Academy of Sciences USA 111:1427-1432.
Group of Fish Reproduction and Culture
IRTA Sant Carles de la Rapita
Contact
Dr. Neil Duncan
neil.duncan@irta.cat
Phone: +34 977 745427 extension 1815
Address
IRTA Sant Carles de la Rapita
Ctra. Poble Nou, km 5.5,
43540 Sant Carles de la Rapita
Tarragona, Spain
Group description
The group is part of the Aquatic Culture Unit in IRTA (http://www.irta.cat) Sant Carles de la Rapita, Tarragona. The group is currently formed by the one group leader (Neil Duncan) and two PhD students (Zohar Ibarra and Elvira Fatsini).
Research topics
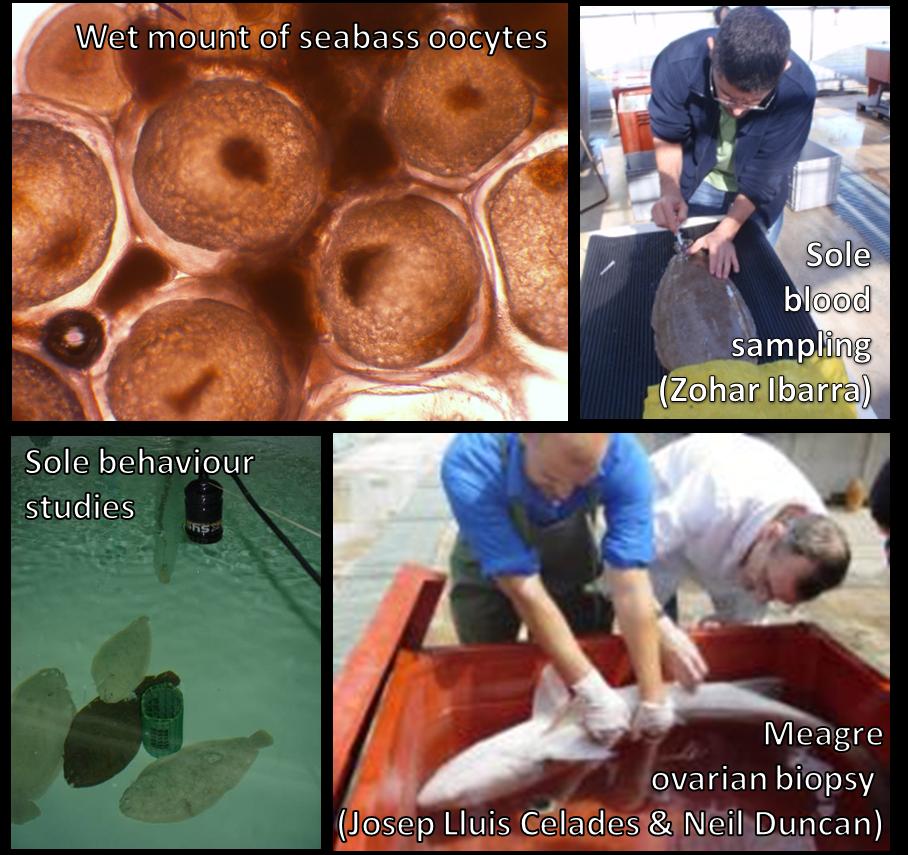
The main research focus of the group is the development of protocols for the aquaculture industry that offer solutions to reproductive dysfunctions and secure the supply of good quality gametes when required and in the quantities required for production purposes. Recent and ongoing research areas are: 1) Development of hormone induction protocols for meagre (Argyrosomus regius) that commonly do not spawn in captivity. 2) Behavioural and nutritional studies of Senegalese sole (Solea senegalensis) broodstock to increase our understanding of the near total reproductive failure in sole reared in captivity. 3) Description and manipulation, with hormone induction, of spawning kinetics and parental contribution of gilthead seabream (Sparus aurata) and European seabass (Dicentrarchus labrax) to aid the development of programs for genetic improvement.
Relevant publications
1. Duncan, N.J., Sonesson, A.K., and Chavanne, H., 2013. Principles of finfish broodstock management in aquaculture: control of reproduction and genetic improvement. In: Allan, G., Burnell, G. (Eds.), Advances in Aquaculture Hatchery Technology. Woodhead Publishing Limited, Cambridge, UK.
2. Mañanos, E., Duncan, N., Mylonas, C., 2008. Reproduction and control of ovulation, spermiation and spawning in cultured fish. In: Methods in Reproductive Aquaculture. Eds. Cabrita, E., Robles, V., Harraez, P., pp. 3-80. CRC Press Taylor and Francis Group, Baca Raton, USA.
3. Norambuena, Fernando; Mackenzie, Simon; Bell, J. Gordon; Callol, Agnes; Estevez, Alicia; Duncan, Neil, 2012. Prostaglandin (F and E, 2- and 3-series) production and cyclooxygenase (COX-2) gene expression of wild and cultured broodstock of senegalese sole (Solea senegalensis). General and Comparative Endocrinology Volume: 177 Issue: 2: 256-262.
4. Duncan, Neil; Estevez, Alicia; Porta, Javier; Carazo, Ignacio; Norambuena, Fernando ; Aguilera, Cristobal ; Gairin, Ignaci; Bucci, Francesco; Valles, Roser; Mylonas, Constantinos C., 2012. Reproductive development, GnRHa-induced spawning and egg quality of wild meagre (Argyrosomus regius) acclimatised to captivity. Fish Physiology and Biochemistry Volume: 38 Issue: 5 Pages: 1273-1286.
5. Oliveira, C., N.J. Duncan, P. Pousão-Ferreira, E. Mañanós, F.J. Sánchez-Vázquez, 2010. Influence of the lunar cycle on plasma melatonin, vitellogenin and sex steroids rhythms in Senegal sole, Solea senegalensis. Aquaculture 306: 343–347.
6. Duncan, N.J., L Ibarra-Castro & R Alvarez-Villaseñor, 2008. Effect of the dusk photoperiod change from light to dark on the incubation period of eggs of the spotted rose snapper, Lutjanus guttatus (Steindachner). Aquaculture Research, 2007, 1-7 doi:10.1111/j.1365-2109.2007.01851.x DATE : 2008.
7. Ibarra-Castro, Leonardo, Neil J. Duncan. GnRHa-induced spawning of wild-caught spotted rose snapper Lutjanus guttatus. Aquaculture 272: 737–746.
8. Agulleiro, Maria J., Alexander P Scott , Neil Duncan , Constantinos C Mylonas , Joan Cerdà, 2007. Treatment of GnRHa-implanted Senegalese sole (Solea senegalensis) with 11-ketoandrostenedione stimulates spermatogenesis and increases sperm motility. Comp Biochem Physiol A Mol Integr Physiol. 147: 885-892.
9. Barrón-Vivanco, B., Duncan, N.J., García-Aguilar, N., Gutiérrez, J., García-Gasca, A., 2005. Effects of LHRHa on the expression of stress-related molecules in the ovary of wild caught Sphoeroides annulatus held in captivity. Journal of Fish Biology 66, 1-7.
10. Komar, C., Turnbull, J.F., Roque, A., Fajer, E., Duncan, N.J., 2004. Effect of the water treatment and aeration on the percentage hatch of demersal, adhesive eggs of the bullseye puffer (Sphoeroides annulatus). Aquaculture, volumen 229: pagina inicial 147 pagina final 158.
11. Gutiérrez, J.N., Duncan, N., Estañol, P.V., García-Gasca, A., 2003. Cyclin B mRNA expression in ovaries from wild and captive bullseye puffer (Sphoeroides annulatus Jenyns, 1842). Journal of Experimental Zoology volumen 298A: pagina inicial 211 pagina final 216.
12. Duncan, N.J., Rodriguez M. de O., G.A., Alok, D., Zohar, Y., 2003. Effects of controlled delivery and acute injections of LHRHa on bullseye puffer fish (Sphoeroides annulatus) spawning. Aquaculture, volumen 218: pagina inicial 625 pagina final 635.
13. Duncan, N.J., Thrush, M.A., Elliott, J.A.K., Bromage, N.R., 2002. Seawater growth and maturation of Atlantic salmon (Salmo salar) transferred to sea at different times during the year. Aquaculture volumen 213: pagina inicial 293 pagina final 309.
14. Duncan, N.J., Mitchell, D., Bromage, N.R., 1999. Post smolt growth and maturation of out-of-season 0+ Atlantic salmon (Salmo salar) reared under different photoperiod. Aquaculture, volumen 177: pagina inicial 61 pagina final 71.
15. Porter, M., Duncan, N., Bromage, N.R., 1999. The use of cage lighting to reduce plasma melatonin in Atlantic salmon (Salmo salar) and its effects on the inhibition of grilsing. Aquaculture, volumen 176: pagina inicial 237 pagina final 244.
Group of Fish Chronobiology
Faculty of Biology
University of Murcia
Contact
Prof. F. Javier Sánchez Vázquez
javisan@um.es
Phone: +34 868 887004
Address
Department of Physiology
Faculty of Biology
University of Murcia
30100-Murcia (Spain)
Group description and research lines
Our fish chronobiology group has been devoted to the study of biological rhythms in fish since 1992. Over this period we have mustered a broad range of multidisciplinary scientific and technological skills, working at the molecular, physiological and behavioural levels, in order to obtain an integrative view of the regulation and function of biological timers in fish We have pioneered research in self-feeding behaviour, the neuroendocrine regulation of reproduction rhythms, the onset of clocks during early development and the study of chronotoxicity in fish. Furthermore, we have bridged the gap between research and industry, converting basic knowledge into direct applications with benefits for aquaculture.
The team has one full Professor (F. Javier Sánchez Vázquez), one Senior Lecturer (F.J. Martinez), one “Juan de la Cierva” research fellow (L.M. Vera), four post-docs (J.F. López-Olmeda, B. Blanco, A. Montoya and N. Villamizar) and three Ph.D. students and one technician.
In the last 5 years, our team has published over 60 primary articles and reviews in scientific journals indexed in SCI, most of them in high ranked journals such as Plos Biology, Plos One, Chronobiol. Int., J. Pineal Res., J. Biol. Rhythms and Aquaculture.
Main fish species
European sea bass, gilthead sea bream, Senegalese sole and zebrafish.
Instituto Español de Oceanografía (IEO). C. O de Santander.
Contact
Olvido Chereguini
o.chereguini@st.ieo.es
Inmaculada Rasines
inma.rasines@st.ieo.es
Phone: +34 942348381
Address
Planta de Cultivos Marinos “El Bocal”
Barrio Corbanera s/n Bocal
39012 Monte, Santander, Spain.
Facilities Description
The marine facilities for experimental aquaculture of fish, “El Bocal”, IEO Santander, belonging to Spanish Institute of Oceanography (IEO), has an area of 2400 m2 that includes a tank house, wet and dry laboratories, storerooms and cold and freezer rooms. The installation has tanks of different volumes (70 l – 62000 l) for egg incubation, larval culture, ongrowing, broodstock and production of live feeds.
Group description and research lines
The research group in fish reproduction, formed by two researchers Olvido Chereguini and Inmaculada Rasines, and three technicians, has extensive experience in the techniques of breeding and larval rearing of marine fish, mainly flatfish, as well as in transferred our results to fish farming companies. Since 2001 we have been working on reproduction, larval rearing and on-growing of Senegalese sole (Solea senegalensis) and common sole (S. solea). Currently, our main lines of research are: development of hormonal induction protocols for induction of ovulation and artificial fertilisation; cryopreservation of sperm, quality of gametes and behavioural studies of Senegalese sole broodstock.
Relevant publications
1. J. Peleteiro, O.Chereguini, R. Cal. 1996. Resultados preliminares de fertilizaciones realizadas con esperma crioconservado de rodaballo. Informe Técnico del Instituto Español de Oceanografía 162, 1- 13.
2. O. Chereguini, R. Cal, C. Dreanno, B. Ogier, M. Suquet, G. Maisse, 1997. Short term storage and cryopreservation of turbot sperm. Aquatic Living Resources 10,151- 155.
3. S. Polo, Chereguini O., Rasines I., García de la Banda I., 1999. Tolerance of turbot Scophthalmus maximus (L., 1758) embryos to the cryoprotectant propane-1,2-diol. Informe Técnico del Instituto Español de Oceanografía 117,1- 9.
4. O. Chereguini, I. García de la Banda, I. Rasines, A. Fernández, 1999. Artificial fertilization in turbot, Scophthalmus maximus (L.): different methods and determination of the optimal sperm-egg ratio. Aquaculture Research 30, 319- 324.
5. M. Suquet, O. Chereguini, M-H Omnes, I. Rasines, Y. Normant, I. Pan Souto I., L. Quemener, 1999. Effect of temperature, volume of ova batches, and addition of a diluent, an antibiotic, oxygen and a protein inhibitor on short-term storage capacities of turbot, Psetta maxima, ova. Aquatic Living Resources 12(4), 239-246.
6. O. Chereguini, I. García de la Banda, I. Rasines, A. Fernández, 2001. Larval growth of turbot, Scophthalmus maximus (L.) produced with fresh and cryopreserved sperm. Aquaculture Research 32,133-143.
7. O. Chereguini, I. García de la Banda, I. Rasines, A. Fernández, 2002.Growth and survival of young turbot (Scophthalmus maximus L.) produced with cryopreserved sperm. Aquaculture Research 33, 637- 641.
8. V. Robles, E. Cabrita, E. Cuñado, O. Chereguini, R. Álvarez, M. P. Herráez, 2002. Effect of vitrification solutions on the hatching rate and the activity of selected enzymes in fish embryos. Cryobiology 45 (3), 260- 261.
9. O. Chereguini, I. García de la Banda, M. Herrera, C. Martínez, M. De la Hera, 2003. Cryopreservation of turbot (Scophthalmus maximus (L.) sperm: fertilization and hatching rates. Aquaculture Research 34, 739- 747.
10. E. Cabrita, O. Chereguini, M. Luna, P. de Paz, M.P. Herráez, 2003.Effect of different treatments on the chorion permeability to DMSO of turbot embryos (Scophthalmus maximus). Aquaculture 221, 593-604.
11. E. Cabrita, V. Robles, O. Chereguini, P. Paz, L. Anel, M.P. Herráez, 2003. DMSO influx in turbot embryos exposed to a vitrification protocol. Theriogenology 60, 463-473.
12. García Alcázar A., Chereguini O., Porta J.M., Porta J., Álvarez Herrero M.C., Abellán Martínez E. 2005. Creación y Caracterización de un banco de esperma de lubina Dicentrarchus labrax (L., 1758) de una población mediterránea. Boletín del Instituto Español de Oceanografía 21 (1-4) ,195-199.
13. Suquet M., Chereguini O., Fauvel C., 2009. Cryopreservation of sperm in turbot (Psetta maxima). In: Methods in Reproductive Aquaculture, 463 -467. CRC Press, Boca Raton USA.
14. I. Rasines, M. Gómez, I. Martin, C. Rodríguez, E. Mañanos, O. Chereguini, 2012. Artificial fertilization of Senegalese sole (Solea senegalensis): Hormone therapy administration methods, timing of ovulation and viability of eggs retained in the ovarian cavity. Aquaculture 326-329,129-135.
15. I. Rasines, M. Gómez, I. Martín, C. Rodríguez, E. Mañanós, O. Chereguini, 2013. Artificial fertilization of Senegalese sole (Solea senegalensis): effect of time of day of hormonal treatment in the induction to ovulation. Aquaculture 392-395, 94-97.
Laboratorio de Reproducción Animal
Departamento de Biología Funcional y Antropología Física
Universitat de València
Contact
Dr. Miguel A. Silvestre
miguel.silvestre@uv.es
Phone: +34 96 354 34 55
Address
Departamento de Biología Funcional y Antropología Física
Universitat de València
C/ Doctor Moliner,50
46100 Burjassot. (Spain)
Group description
The Laboratory of Animal Reproduction is a part of the Department of Functional Biology and Physical Anthropology at the Universitat de València (www.uv.es). The research group is formed by 4 tenured university professors and researchers and several ph-D students. The main objective of the group is the study of reproduction, in particular in andrology area, both in fishes as mammals.
The professors teach several subjects (Endocrinology and Reproduction, Biology, …) on several university degrees as Biology or Biotechnology.
Main lines of research
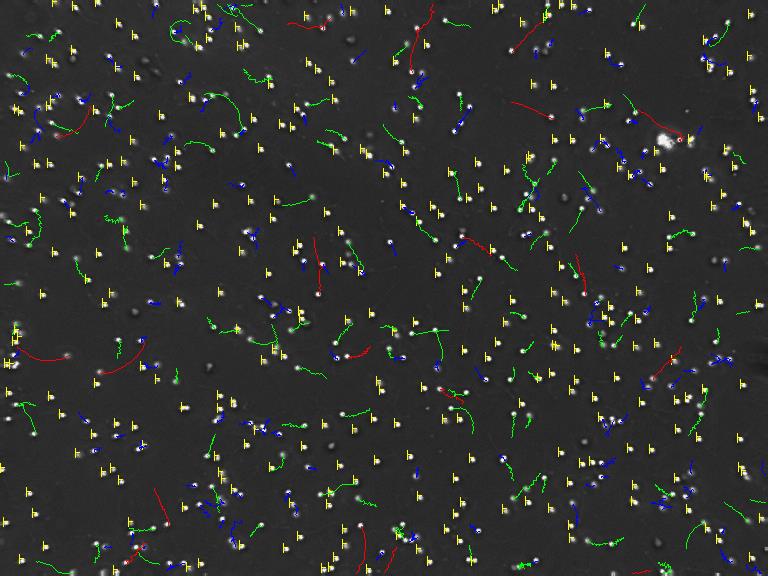
– Activation and Motility of zebrafish sperm.
– Evaluation of conditions of sperm motility patterns, morphology and DNA fragmentation in fishes and mammals.
– Cooled conservation of sperm in fishes and mammals.
– Optimization of CASA system protocols.
Links
Department of Functional Biology and Physical Anthropology: http://www.uv.es/uvweb/functional_biology_physical_anthropology_department/en/organisation/department-governance-structure-1285854909234.html
Relevant publications
1. V Gallego, PC Carneiro, I Mazzeo, MC Vílchez, DS Peñaranda, C Soler, L Pérez, JF Asturiano. 2013. Standardization of European eel (Anguilla anguilla) sperm motility evaluation by CASA software. Theriogenology,79 (7) 1034-1040.
2. JL Yániz, S Capistrós, S Vicente-Fiel, C Soler, M Núñez de Murga, P Santolaria. 2013. Use of Relief Contrast(®) Objective to Improve Sperm Morphometric Analysis by Isas(®) Casa System in the Ram. Reprod Domest Anim, 48, 1019-1024.
3. S Vicente-Fiel, I Palacín, P Santolaria, CO Hidalgo, MA Silvestre, F Arrebola, JL Yániz. 2013. A comparative study of the sperm nuclear morphometry in cattle, goat, sheep, and pigs using a new computer-assisted method (CASMA-F). Theriogenology, 79 (3) 436-442.
4. IA Butts, EA Trippel, A Ciereszko, C Soler, M Słowińska, SM Alavi, MK Litvak, I Babiak. 2011. Seminal plasma biochemistry and spermatozoa characteristics of Atlantic cod (Gadus morhua L.) of wild and cultivated origin. Comp Biochem Physiol, Part A Mol Integr Physiol, 159 (1) 16-24.
5. IA Butts, I Babiak, A Ciereszko, MK Litvak, M Słowińska, C Soler, EA Trippel. 2011. Semen characteristics and their ability to predict sperm cryopreservation potential of Atlantic cod, (Gadus morhua L.). Theriogenology, 75 (7) 1290-1300.
6. I Salvador, JL Yániz, MP Viudes de Castro, EA Gómez, MA Silvestre. 2006. Effect of solid storage on caprine semen conservation at 5 degrees C. Theriogenology, 66 (4), 974-981.
7. F Marco-Jimenez, MP Viudes de Castro, S Balasch, E Mocé, MA Silvestre, EA Gomez, JS Vicente. 2006. Morphometric changes in goat sperm heads induced by cryopreservation. Cryobiology 52 (2), 295-304.
8. C Soler, B Gadea, AJ Soler, MR Fernández-Santos, MC Esteso, J Núñez, PN Moreira, M Núñez, R Gutiérrez, M Sancho, JJ Garde. 2005. Comparison of three different staining methods for the assessment of epididymal red deer sperm morphometry by computerized analysis with ISAS. Theriogenology, 64 (5),1236-1243.
9. JL Yániz, JI Martí, MA Silvestre, J Folch, P Santolaria, JL Alabart, F López-Gatius. 2005. Effects of solid storage of sheep spermatozoa at 15 degrees C on their survival and penetrating capacity. Theriogenology, 64, 1844-1851.
10. C Soler, JJ de Monserrat, M Nunez, R Gutierrez, J Nunez, M Sancho, TG Cooper. 2005. Regionalization of epididymal duct and epithelium in rats and mice by automatic computer-aided morphometric analysis. Asian J Androl, 7 (3), 267-275.
11. F López-Gatius, G Sances, M Sancho, JL Yániz, P Santolaria, A Gutierrez-Adan, M Núñez, J Núñez, C Soler. 2005. Effect of solid storage at 15 degrees C on the subsequent motility and fertility of rabbit semen. Theriogenology, 64, 252-260.
12. C Soler, JJ de Monserrat, R Gutiérrez, J Nuñez, M Nuñez, M Sancho, F Pérez-Sánchez, TG Cooper. 2003. Use of the Sperm-Class Analyser for objective assessment of human sperm morphology. Int J Androl 26 (5) 262-270.
13. C Gago, F Pérez-Sánchez, CH Yeung, L Tablado, TG Cooper, C Soler. 1998. Standardization of sampling and staining methods for the morphometric evaluation of sperm heads in the Cynomolgus monkey (Macaca fascicularis) using computer-assisted image analysis. Int J Androl 21 (3), 169-176
14. F Pérez-Sánchez, L Tablado, C Soler. 1997. Sperm morphological abnormalities appearing in the male rabbit reproductive tract. Theriogenology 47 (4) 893-901
15. F Pérez-Sánchez, L Tablado, CH Yeung, TG Cooper, C Soler. 1996. Changes in the motility patterns of spermatozoa from the rabbit epididymis as assessed by computer-aided sperm motion analysis. Mol. Reprod. Dev. 45 (3) 364-371.
Grupo de Biología Celular en Toxicología Ambiental (BCTA-UPV/EHU)
http://www.ehu.es/es/web/cellbiologyinenvironmentaltoxicology
Contact
Dr. Ibon Cancio
Ibon.cancio@ehu.es
Phone: +34 946012734
Address
Dept of Zoology and Animal Cell Biology
Plentzia Marine Station (PIE UPV/EHU)
University of the Basque Country (UPV/EHU)
Areatza s/n, E-48260 Plentzia
Group description
Since 1985 the group of “Cell Biology in Environmental Toxicology” of the University of the Basque Country develops research and teaching activities within the Environmental Toxicology field (both aquatic and terrestrial ecosystems). The group, recognized since 2001 as consolidated research group, is composed of 10 university lecturers and researchers, 3 postdoc, 13 PhD students, 1 lab technician and 1 administrative. The group´s lab contains facilities for histology, light, fluorescence and electron microscopy, image analysis, cell fractionation and biochemistry, cell culture and molecular biology. The main field of expertise of the group is in development of early warning cell and molecular markers of pollution exposure and effects. The group has developed a novel biomarker, peroxisome proliferation, useful as exposure biomarker of PAHs, PCBs, phthalate esters, pesticides etc, recently adopted by different European labs and implemented in international programmes such as UNEP´s MEDPOL. The group also developed a battery of toxicity tests in vitro with mussel haemocytes as marker of immunotoxicity and a second battery with mussel gill cells. The group measures routinely several well-established biomarkers of pollution, participating in intercalibration trials (BEEP, BEQUALM, UNEP-MEDPOL). Recently the group incorporated the proteomic approach, to search for protein expression signatures as novel integrated biomarkers. The group is also investigating changes in gene transcription after cloning of several target genes in different pollution sentinel species (mainly fish) and is interested in development and application of microarrays. Special areas of interest are the elucidation of mechanisms of chemical carcinogenesis, reproductive endocrine disruption and nuclear receptors (PPARs, ER, RXR). The group has been involved in monitoring of biological effects of pollution along the Basque coast, Mediterranean coast and North Sea.
Since July 2012, the research group manages and works in the Plentzia Marine Station (http://www.ehu.es/PIE/), which was created due to the initiative of research group members. This infrastructure belonging to the University is a 2500 m2 building with 750 m2 of aquaria facilities, situated on the beach of the touristic town of Plentzia.
Members of the research group coordinate two official masters one in “Environmental Contamination and Toxicology” belonging to the UPV/EHU and the other one in conjunction with the Universities of Southampton, Bordeaux and Liege in “Marine Environmental Resources” (Erasmus-Mundus Master). Both masters are hosted in the Plentzia Marine Station.
Main lines of research in relation to fish gametes
– Environmental reproductive endocrine disruption:
Xenostrogenicity, intersex, vitellogenin, aromatases, GSI index, ALP in molluscs, environmental monitoring of exposure to endocrine disruptors, fish embryo toxicity, transcriptional profiles in fish and mollusc gonads.
– Fish sexual differentiation
Regulation of of oogenesis, oocyte differentiation and maturation, vitellogenesis
– Fisheries and aquaculture
Fish fecundity, oocyte quality traits, molecular markers of sex and oocyte quality
Relevant publications
1.- Bilbao, E. Raingeard, D. Diaz de Cerio, O. Ortiz-Zarragoitia, M. Ruiz, P. Izagirre, U. Orbea, A. Marigómez, I. Cajaraville, M.P. & Cancio, I. “Effects of exposure to Prestige-like heavy fuel oil and to perfluorooctane sulfonate on conventional biomarkers and target gene transcription of the thicklip grey mullet Chelon labrosus.” AQUAT. TOXICOL. 98: 282-296 (2010).
2.- Ortiz-Zarragoitia M, & Cajaraville MP. “Intersex and oocyte atresia in a mussel population from the Biosphere’s Reserve of Urdaibai (Bay of Biscay)”. ECOTOXICOL ENVIRON SAF. 73: 693-701 (2010).
3.- Puy-Azurmendi E, Ortiz-Zarragoitia M, Kuster M, Martínez E, Guillamón M, Domínguez C, Serrano T, Barbero MC, Alda ML, Bayona JM, Barceló D, & Cajaraville MP. “An integrated study of endocrine disruptors in sediments and reproduction-related parameters in bivalve molluscs from the Biosphere’s Reserve of Urdaibai (Bay of Biscay)”. MAR ENVIRON RES. 69 Suppl: S63-6 (2010).
4.- Baussant T, Ortiz-Zarragoitia M, Cajaraville MP, Bechmann RK, Taban IC, & Sanni S. “Effects of chronic exposure to dispersed oil on selected reproductive processes in adult blue mussels (Mytilus edulis) and the consequences for the early life stages of their larvae”. MAR POLLUT BULL. 62: 1437-1445 (2011).
5.- Ortiz-Zarragoitia M, Garmendia L, Barbero MC, Serrano T, Marigómez I, & Cajaraville MP. “Effects of the fuel oil spilled by the Prestige tanker on reproduction parameters of wild mussel populations”. J ENVIRON MONIT. 13: 84-94 (2011).
6.- Brooks S;., Harman, C; Soto, M; Cancio, I; Glette, T. & Marigómez, I. “Integrated coastal monitoring of a gas processing plant using native and caged mussels” SCI. TOT. ENVIRON. 426: 375–386 (2012).
7.- Filby AL, Paull GC, Searle F, Ortiz-Zarragoitia M, & Tyler CR. “Environmental estrogen-induced alterations of male aggression and dominance hierarchies in fish: a mechanistic analysis”. ENVIRON SCI TECHNOL. 46: 3472-3479 (2012).
8.- Diaz de Cerio, O; Rojo-Bartolomé, I; Bizarro, C; Ortiz-Zarragoitia, M. & Cancio, I. “5S rRNA and accompanying proteins in gonads: powerful markers to identify sex and reproductive endocrine disruption in fish” ENVIRON. SCI. TECHNOL. 46: 7763-7771 (2012).
9.- Humble J.L; Hands E; Saaristo, M; Lindström, K; Lehtonen, K; Diaz de Cerio, O; Cancio, I; Wilson, G. & Craft, JA. “Characterisation of genes transcriptionally upregulated in the liver of sand goby (Pomatoschistus minutus) by 17α-ethinyloestradiol: identification of distinct vitellogenin and zona radiata protein transcripts” CHEMOSPHERE. 90: 2722-2729 (2013).
10.- Raingeard D, Bilbao E, & Cancio I, “Retinoid X receptor (RXR), estrogen receptor (ER) and other nuclear receptors in tissues of the mussel Mytilus galloprovincialis: cloning and transcription pattern”. COMP BIOCHEM PHYSIOL 165A: 178-190 (2013).
11.- Puy-Azurmendi E, Ortiz-Zarragoitia M, Villagrasa M, Kuster M, Aragón P, Atienza J, Puchades R, Maquieira A, Domínguez C, López de Alda M, Fernandes D, Porte C, Bayona JM, Barceló D, & Cajaraville MP. “Endocrine disruption in thicklip grey mullet (Chelon labrosus) from the Urdaibai Biosphere Reserve (Bay of Biscay, Southwestern Europe)”. SCI TOTAL ENVIRON. 443: 233-244 (2013).
12.- Marigómez I, Zorita I, Izagirre U, Ortiz-Zarragoitia M, Navarro P, Etxebarria N, Orbea A, Soto M, & Cajaraville MP. “Combined use of native and caged mussels to assess biological effects of pollution through the integrative biomarker approach”. AQUAT TOXICOL. 136-137: 32-48 (2013).
13.- Puy-Azurmendi E, Olivares A, Vallejo A, Ortiz-Zarragoitia M, Piña B, Zuloaga O, & Cajaraville MP. “Estrogenic effects of nonylphenol and octylphenol isomers in vitro by recombinant yeast assay (RYA) and in vivo with early life stages of zebrafish”. SCI TOTAL ENVIRON. 466-467: 1-10 (2014).
14.- Bizarro C, Ros O, Vallejo A, Prieto A, Etxebarria N, Cajaraville MP, & Ortiz-Zarragoitia M. “Intersex condition and molecular markers of endocrine disruption in relation with burdens of emerging pollutants in thicklip grey mullets (Chelon labrosus) from Basque estuaries (South-East Bay of Biscay)”. MAR ENVIRON RES. 96: 19-28 (2014).
15.- Ortiz-Zarragoitia, M.; Bizarro, C; Rojo-Bartolomé, I; Diaz de Cerio, O; Cajaraville MP. & Cancio, I. “Mugilid fish: sentinels of exposure to endocrine disrupting compounds in littoral and estuarine waters and research models for the discovery of molecular markers of intersex condition” MAR. DRUGS (sent for publication)
Grupo de Control de Ingesta (CDI)
Instituto de Acuicultura de Torre de la Sal
Consejo Superior de Investigaciones Científicas
Contact
Jose Miguel Cerda-Reverter
E-mail: jm.cerda.reverter@csic.es
Phone: +34964319500 ext 39
Address
Instituto de Acuicultura de Torre de la Sal (IATS)
Consejo Superior de Investigaciones Científicas (CSIC)
Torre de la Sal s/n, Ribera de Cabanes, 12595 Castellon, SPAIN
Group Description
The group “Control of Food Intake” is part of the Department of Fish Physiology at the Institute of Aquaculture from Torre de la Sal (IATS). The group is now formed by 1 Research Scientist 1 Professor, 1 technician and 3 PhD students.
Main Research Lines
– Neural control of food intake and energy balance in teleost fish with particular emphasis in the melanocortin system
– Crosstalk between stress-energy balanced and growth. Role of the melanocortin system
– Relation between the regulation of energy balance and reproduction with particular emphasis in puberty
Relevant publications
1. Cerdá-Reverter JM, Sorbera L, Carrillo M and Zanuy S. Energetic Dependence of NPY-Induced LH Secretion in a Teleost Fish (Dicentrarchus labrax). American Journal of Physiology 277, Integrative Comparative Physiology 46: R1627 -R1634, 1999.
2. Cerdá-Reverter JM, Ringholm A, Schiöth HB and Peter RE. Molecular cloning, pharmacological characterization and brain mapping of the melanocortin 4 receptor in the goldfish: Involvement in the control of food intake. Endocrinology 144: 2336-2349, 2003. DOI: 10.1210/en.2002-0213.
3. Cerdá-Reverter JM and Peter RE. Endogenous melanocortin antagonist in fish. Structure, brain mapping and regulation by fasting of the goldfish agouti-related protein gene. Endocrinology 144: 4552-4561, 2003.DOI: 10.1210/en.2003-0453.
4. Cerdá-Reverter JM, Ling M, Schiöth HB and Peter RE. Molecular cloning, pharmacological characterization and brain mapping of the melanocortin 5 receptor in the goldfish. Journal of Neurochemistry, 87:1354-1367, 2003. DOI: 10.1046/j.1471-4159.2003.02107.x.
5. Cerdá-Reverter JM, Haitina T, Schiöth HB and Peter RE. Gene structure of the goldfish agouti-signaling protein: a putative role in the dorsal-ventral pigment pattern of fish. Endocrinology, 146:1597-1610, 2005. DOI: 10.1210/en.2004-1346.
6. Volkoff H, Canosa LF, Unniappan S, Cerdá-Reverter JM, Bernier NJ, Kelly SP, and Peter RE. Neuropeptides and the control of food intake in fish. General Comparative Endocrinology, 142:3-19, 2005. DOI: 10.1016/j.ygcen.2004.11.001. (IF-2005: 2,290, Q3).
7. Cerdá-Reverter JM, Canosa LF, Peter RE. Regulation of the hypothalamic melanin-concentrating hormone neurons by sex steroids in the goldfish: possible role in the modulation of luteinizing hormone secretion. Neuroendocrinology, 84:364-77, 2006. DOI: 10.1159/000098334.
8. Sánchez E, Rubio VC, Thompson D, Metz J, Flik G, Millhauser GL, Cerdá-Reverter JM. Phosphodiesterase inhibitor-dependent inverse agonism of agouti-related protein (AGRP) on melanocortin 4 receptor in sea bass (Dicentrarchus labrax). American Journal of Physiology, Regulatory, Integrative and Comparative Physiology, 296: R1293-R1306, 2009. DOI: 10.1152/ajpregu.90948.2008. 29.
9. Leal E, Sánchez E, Muriach B, Cerdá-Reverter JM. Sex Steroid-Induced Food Intake Inhibition in the Sea Bass (Dicentrarchus labrax). Journal of Comparative Physiology B, 179:77 – 86, 2009. DOI: 10.1007/s00360-008-0285-5.
10. Agulleiro MJ, Roy S, Sánchez E, Puchol S, Gallo-Payet N, Cerdá-Reverter JM. Role of accessory proteins in the function of zebrafish melanocortin receptor type 2. Molecular and Cellular Endocrinology, 320: 145-152, 2010. DOI: 10.1016/j.mce.2010.01.032.
11. Agulleiro MJ, Cortés R, Fernández-Durán B, Guillot R, Navarro S, Meimaridou E, Clark AJ, Cerdá-Reverter JM Melanocortin 4 receptor becomes an ACTH receptor by coexpression of melanocortin receptor accessory protein 2. Molecular Endocrinology, 27:1934-1945. 2013. DOI: 10.1210/me.2013-1099. 46.
12. Leal E, Fernández-Durán B, Agulleiro MJ, Conde-Siera M, Míguez JM, Cerdá-Reverter JM. Effects of dopaminergic system activation on feeding behavior and growth performance of the sea bass (Dicentrarchus labrax): a self-feeding approach. Hormones and Behavior, 64: 113-121, 2013. DOI: 10.1016/j.yhbeh.2013.05.008. 46.
13. Guillot R, Ceinos R, Cal R, Rotllant J, Cerdá-Reverter JM. Transient ectopic overexpression of agouti-signalling protein 1 (asip1) induces pigment anomalies in flatfish. PLoS ONE 7(12): e48526, 2012. DOI: 10.1371/journal.pone.0048526.
14. Agulleiro MJ, Sánchez E, Leal E, Cortés R, Fernández-Durán B, Guillot R, Davis P, Dores RM, Gallo-Payet N, Cerdá-Reverter JM. Molecular Characterization and Functional Regulation of Melanocortin Receptor Type 2 (MC2R) in the Sea Bass (Dicentrarchus labrax). A putative role in the adaptation to stress. PloS ONE 8(5):e65450, 2013. DOI: 10.1371/journal.pone.0065450. 45.
15. Ceinos RM, Guillot R, Kelsh RN, Cerdá-Reverter JM, Rotllant J. Pigment patterns in adult fish result from superimposition of two largely independent pigmentation mechanisms. Pigment Cell and Melanoma Research : 28:196-209. doi: 10.1111/pcmr.12335.