Aquaculture Research Group
Centre of Marine Sciences (CCMAR)
Contact
Dr. Maria Teresa Dinis
mtdinis@ualg.pt
Phone: +351 289 800 900 ext. 7927
Address
Centro de Ciências do Mar do Algarve (CCMAR)
Universidade do Algarve
Campus de Gambelas
8005-139 Faro
Portugal
Group description
The Aquaculture Research Group is part of the Centre of Marine Sciences (CCMAR), a non-profit organization located on the Gambelas campus of the University of Algarve and dedicated to R&D in the Marine Sciences. On the basis of international evaluation and excellence ranking CCMAR was awarded the status of Associate Laboratory together with CIIMAR (Interdisciplinary Centre for Marine and Environmental Research). It has 205 people on its staff, 88 and 114 of which hold a PhD and college degree, respectively.
The centre counts with laboratories and fish facilities for larval, juvenile and broodstock.
The aim of the Aquaculture Research Group is the generation of scientific knowledge, through basic and applied research, for the promotion of the sustainable development of the aquaculture industry. The group is comprised by 10 researchers with a PhD degree, in addition to a fluctuating number of guest researchers and students.
The group has expertise in several marine and fresh water fish species (gilthead sea bream, European sea bass, trout, dusky grouper, Senegalese sole and meagre).
Senegalese sole Epinephelus marginatus Sparus aurata
The main skills of the group include fish gametes quality evaluation and cryopreservation; phyto- and zooplankton production; tracer studies for study of nutrient utilization; larval rearing, feeding and nutrition of several marine fish species. These activities have been developed in the framework of several research projects over the last ten years, at both national and international level (e.g., PRAXIS XII, POCTI, INTERREG, STRIDE, AIR, FAIR, CRAFT and QLM programs).
The group also has a long collaboration on joint supervision of PhD theses with European institutions, participation in European research networks (COST, EATIP) and as partner in projects involving an industrial collaboration.
Main lines of research
Nutrition for enhanced performance and quality
o Determination of metabolic nutrient utilization in marine fish larvae and juveniles;
o Broodstock management (including sperm quality and cryopreservation);
o Fish safety and quality.
Sustainable practices
o Production systems for new species;
o Fish welfare.
Links
![]() Centre of Marine Sciences (CCMAR): www.ccmar.ualg.pt
Centre of Marine Sciences (CCMAR): www.ccmar.ualg.pt
![]() Aquaculture Research Group: http://www.ccmar.ualg.pt/aquagroup/
Aquaculture Research Group: http://www.ccmar.ualg.pt/aquagroup/
Relevant publications
1. Alves, R.N., Cordeiro, O., Silva, T.S., Richard, N., de Vareilles, M., Marino, G., Di Marco, P., Rodrigues, P.M., Conceicao, L.E.C., 2010. Metabolic molecular indicators of chronic stress in gilthead seabream (Sparus aurata) using comparative proteomics. Aquaculture. 299, 57-66.
2. Conceição, L., Aragão, C., Richard, N., Engrola, S., Gavaia, P., Mira, S., Dias, J., 2010. Novel methodologies in marine fish larval nutrition. Fish Physiology and Biochemistry. 36, 1-16.
3. Conceição, L.E.C., Aragão, C., Rønnestad, I., 2011. Proteins. In: Holt, J. (Ed.), Larval Fish Nutrition, Inc. UK, pp. 83-116.
4. Cordeiro, O., Silva, T., Alves, R., Costas, B., Wulff, T., Richard, N., de Vareilles, M., Conceição, L., Rodrigues, P., 2012. Changes in liver proteome expression of Senegalese sole (Solea senegalensis) in response to repeated handling stress. Marine Biotechnology. 14, 714-729.
5. Engrola, S., Dinis, M.T., L.E.C., C., 2010. Senegalese sole larvae growth and protein utilization is depressed when co-fed high levels of inert diet and Artemia since first feeding. Aquaculture Nutrition. 16, 457-465.
6. Engrola, S., Mai, M., Dinis, M.T., Conceição, L.E.C., 2009. Co-feeding of inert diet from mouth opening does not impair protein utilization by Senegalese sole larvae. Aquaculture. 287, 185-190.
7. Martínez-Páramo, S., Diogo, P., Beirão, J., Dinis, M.T., Cabrita, E., 2012a. Sperm lipid peroxidation is correlated with differences in sperm quality during the reproductive season in precocious European sea bass (Dicentrarchus labrax) males. Aquaculture. 358–359, 246-252.
8. Martínez-Páramo, S., Diogo, P., Dinis, M.T., Herráez, M.P., Sarasquete, C., Cabrita, E., 2012b. Incorporation of ascorbic acid and α-tocopherol to the extender media to enhance antioxidant system of cryopreserved seabass sperm. Theriogenology. 77, 1129-1136.
9. Martínez-Páramo, S., Diogo, P., Dinis, M.T., Herráez, M.P., Sarasquete, C., Cabrita, E., 2012c. Sea bass sperm freezability is influenced by motility variables and membrane lipid composition but not by membrane integrity and lipid peroxidation. Animal reproduction science. 131, 211-218.
10. Martínez-Páramo, S., Diogo, P., Dinis, M.T., Soares, F., Sarasquete, C., Cabrita, E., 2013. Effect of two sulfur-containing amino acids, taurine and hypotaurine in European sea bass (Dicentrarchus labrax) sperm cryopreservation. Cryobiology. 66, 333-338.
11. Oliveira, C., Mañanós, E., Ramos, J., Sánchez-Vázquez, F.J., 2011. Impact of photoperiod manipulation on day/night changes in melatonin, sex steroids and vitellogenin plasma levels and spawning rhythms in Senegal sole, Solea senegalensis. Comparative Biochemistry and Physiology – Part A: Molecular & Integrative Physiology. 159, 291-293.
12. Oliveira, C., Dinis, M.T., Soares, F., Cabrita, E., Pousão-Ferreira, P., Sanchez-Vazquez, F.J., 2009. Lunar and daily spawning rhythms of Senegal sole Solea senegalensis. Journal of Fish Biology. 75, 61-74.
13. Pérez-Cerezales, S., Martínez-Páramo, S., Beirão, J., Herráez, P., 2010. Fertilization capacity with rainbow trout DNA damaged sperm and embryo developmental success. Reproduction. 139, 1-10.
14. Pinto, W., Rønnestad, I., Olderbakk Jordal, A.-E., Gomes, A.S., Dinis, M.T., Aragão, C., 2012. Cloning, tissue and ontogenetic expression of the taurine transporter in the flatfish Senegalese sole (Solea senegalensis). Amino acids 42, 1317-1327.
15. Rodrigues, P.M., Silva, T.S., Dias, J., Jessen, F., 2012. PROTEOMICS in aquaculture: Applications and trends. Journal of Proteomics. 75, 4325-4345.
Group of Molecular Biology of Marine Organisms (EDGE)
Centre of Marine Sciences (CCMAR)
University of Algarve
Contact
Dr. Paulo J. Gavaia
pgavaia@ualg.pt
Phone: +351.289.800057
Address
Centro de Ciências do Mar (CCMAR)
Edifício 7, Campus de Gambelas
University of Algarve
8005-139 Faro, Portugal
Group description
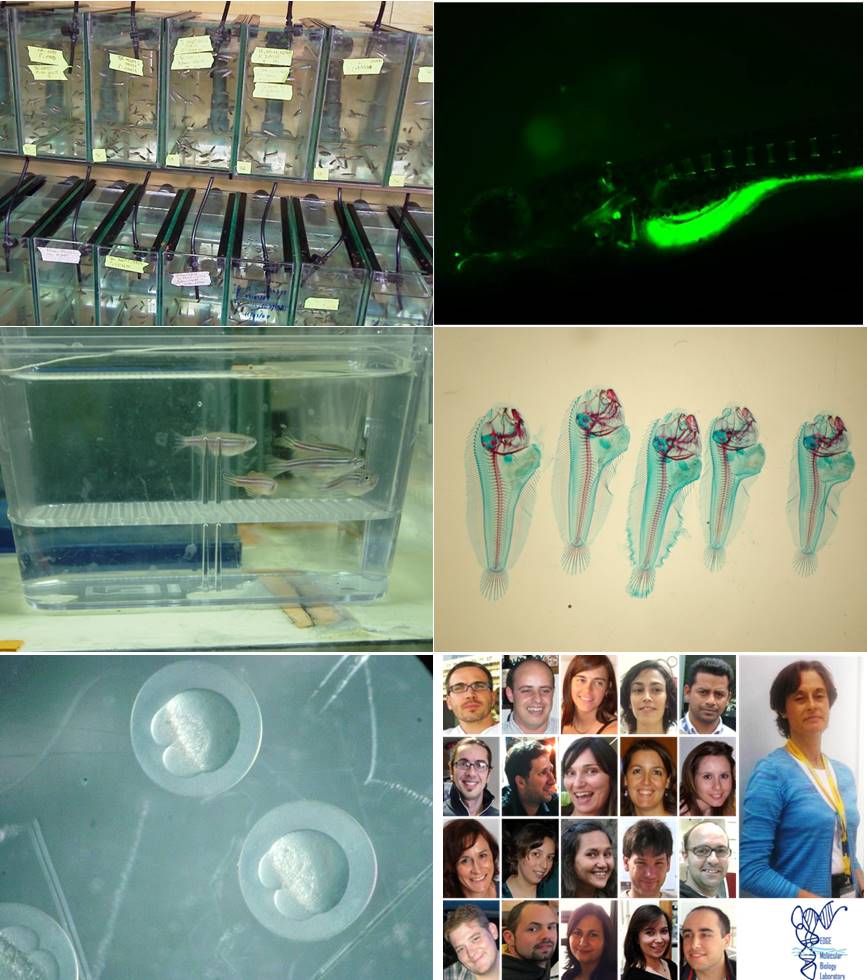
The group of Molecular Biology of Marine Organisms (EDGE) belongs to the Centre of Marine Sciences (CCMAR), located at the Campus de Gambelas of the University of Algarve in Faro, Portugal. Our group consists of 1 full Professor, 2 associated Researchers, 3 post-docs, 12 PhD students and several technicians, MSc students and undergraduate students. Most of the lab members are working in different aspects of skeletal biology of vertebrates and on the gene expression and regulation, either using model animals (zebrafish, marine teleosts, mouse and rat) or in vitro tools like the fish derived osteo-chondroblastic cell lines or the blastema and embryonic stem-like cell cells developed in our laboratory. Different lines of research are currently being followed by researchers in EDGE group, supported also by an excellent collaborative network involving companies and researchers from several institutions belonging to countries in Europe (11), Americas (3), Oceania (1) and Asia (1).
Main lines of research
Molecular determinants of extracellular matrix calcification. Research focus is primarily on understanding molecular pathways of tissue mineralization and its regulation in adult life and during development, using teleost, cartilaginous fish and amphibians as model organisms; additional goals include studies on (1) the molecular adaptations of mechanisms that control extracellular matrix mineralization, (2) the evolution of mineralization-related gene structure and protein function and (3) the mechanisms of mineralization in mollusc bivalves. Goals include (1) the use of integrated multidisciplinary approaches for in vivo / in vitro functional analysis of bone- and cartilage-related genes through techniques of overexpression and RNA interference, cell line and transgenic zebrafish development, (2) evolutionary studies on specific gene function and environmental adaptations affecting bone biology and (3) function of mineralization-related proteins from mollusc bivalves.
Zebrafish development and nutrition. Research is focused on the effects of nutritional factors on the mechanisms regulating zebrafish development, reproduction, osteogenesis and osteotoxicity. The main goal is to establish nutritional requirements at different life stages in order to develop a standardized diet for zebrafish and to test different micronutrients and molecules in order to maximize reproductive success and improve gamete quality, larval survival, early development and osteogenesis. An ongoing collaboration with the Aquagroup from the same research centre aims at developing novel strategies and procedures for zebrafish germ cell cryopreservation and development of molecular tools for sperm quality evaluation.
Relevant publications
1. Gavaia, P.J., Simes, D.C., Viegas C.S.B., Pinto J., Ortiz-Delgado J.B., Kelsh R.N., Sarasquete M.C., Cancela M.L. 2006. Osteocalcin and Matrix Gla Protein in zebrafish (Danio rerio) and Senegal sole (Solea senegalensis): comparative gene and protein expression during larval development through adulthood. Mechanisms of Development- Gene Expression Patterns 6(6):637-52. doi:10.1016/j.modgep.2005.11.010
2. Vânia P. Roberto, Sofia Cavaco, Carla S.B. Viegas, Dina C Simes, Juan-Bosco Ortiz, Carmen Sarasquete, Paulo J. Gavaia, M. Leonor Cancela. 2009. Matrix Gla protein in turbot (Scophthalmus maximus): Gene expression analysis and identification of sites of protein accumulation. Aquaculture 294, 202-211. doi:10.1016/j.aquaculture.2009.06.020
3. Gavaia P.J., Domingues S.S., Engrola S., Drake P., Sarasquete C., M.T. Dinis, Cancela M.L. 2009. Comparing skeletal development of wild and hatchery-reared Senegalese sole (Solea senegalensis, Kaup 1858): evaluation in larval and postlarval stages. Aquaculture Research 40 (14), 1585-1593. doi:10.1111/j.1365-2109.2009.02258.x
4. Conceição, L.E.C., Aragão, C., Richard, N., Engrola, S., Gavaia, P.J., Mira, S., Dias, J. 2010. Novel methodologies in marine fish larval nutrition. Fish Physiology and Biochemistry 36:1-16. doi:10.1007/s10695-009-9373-z
5. Dionísio, G., Campos, C., Valente, L.M.P., Conceição L.E.C., Cancela, M.L., Gavaia, P.J. 2012 .Effect of egg incubation temperature on the occurrence of skeletal deformities in Solea senegalensis. Journal of Applied Ichthyology 28: 471–476. doi: 10.1111/j.1439-0426.2012.01996.x
6. Parameswaran V., Laizé V., Gavaia P.J., Cancela M.L. 2012. ESSA1 embryonic stem like cells from gilthead seabream: A new tool to study mesenchymal cell lineage differentiation in fish. Differentiation 84: 240-251 doi:10.1016/j.diff.2012.07.004
7. Parameswaran, V, Laizé, V, Cardeira, J, Trindade, M, Cancela, ML. 2013. Development of an in vitro cell system from zebrafish suitable to study bone cell differentiation and extracellular matrix mineralization. Zebrafish 10: 500-509 doi: 10.1089/zeb.2012.0833
8. Pacchiarini T., Cross I., Leite R., Gavaia P., Ortiz-Delgado J.B., Pousão-Ferreira P., Rebordinos L., Sarasquete C., Cabrita E. 2013. The Solea senegalensis vasa homologues: molecular characterization, tissue distribution and developmental expression profiles. Reproduction, Fertility and Development 25 (4), 646-660. doi: 10.1071/RD11240
9. Boglione C., Gavaia P., Koumoundouros G., Gisbert E., Moren M., Fontagné S., Witten P. E. 2013. A review on skeletal anomalies in reared European larvae and juveniles. Part 1: Normal and anomalous skeletogenic processes. Reviews in aquaculture 5, S99-S120. DOI:10.1111/raq.12015
10. Boglione C., Gisbert E., Gavaia P., Witten P. E., Moren M., Fontagné S., Koumoundouros G. 2013. A review on skeletal anomalies in reared European larvae and juveniles. Part 2: Main typologies, occurrences and causative factors. Reviews in aquaculture 5, S121-S167. doi:10.1111/raq.12016
11. Cunha M.E., Ré P., Ferreira H.Q., Gavaia P.J., Pousão-Ferreira P. 2013. Larval and juvenile development of dusky grouper, Epinephelus marginatus (Lowe, 1834), reared in mesocosm. J. Fish Biol. 83(3):448-65. doi:10.1111/jfb.12180
12. Rocha F., Dias J., Engrola S., Gavaia P., Geurden I., Dinis M.T., Panserat S. 2014. Glucose overload in yolk has little effects on the long term modulation of carbohydrate metabolic genes in zebrafish (Danio rerio). J Exp. Biol. 217:1139-1149 doi:10.1242/jeb.095463
13. Richard N., Fernández I., Wulff T., Hamre K., Cancela M.L., Conceição L., Gavaia P.J. 2014. Dietary supplementation with vitamin K affects transcriptome and proteome of Senegalese sole, improving larval performance and quality. Marine Biotechnology, in press doi:10.1007/s10126-014-9571-2
14. Cabrita E., Martínez-Páramo S., Gavaia P.J., Riesco M.F., Valcarce D.G., Sarasquete C., Herráez M.P., Robles V. 2014. Factors enhancing fish sperm quality and emerging tools for sperm analysis. Aquaculture In press doi: http://dx.doi.org/10.1016/j.aquaculture.2014.04.034
15. Diogo P., Martins G., Gavaia P.J., Pinto W., Dias J., Cancela L., Martínez-Páramo S. 2014. Assessment of nutritional supplementation in phospholipids on the reproductive performance of zebrafish (Danio rerio). Accepted to Journal of Applied Ichthyology.