Gruppo di Fisiologia Comparata
DiSTeBA
Università degli Studi del Salento
Contact
Sebastiano VILELLA
Associate Professor of Physiology
e-mail: sebastiano.vilella@unisalento.it
Tel: +39 0832 29 8671
Address
Laboratory of Comparative Physiology
Department of biological and environmental sciences and technology
University of Salento, 73100 Lecce, Italy
Group description
The group is formed by 1 professor , 1 researcher, 1 technician and 1 Ph D student. The group works on: reproductive physiology of teleost fish (quality of gametes, mechanisms of motility activation and effect of cryo-preservation on gametes quality, hormonal treatments for sexual maturation and spawning); fish nutrition.
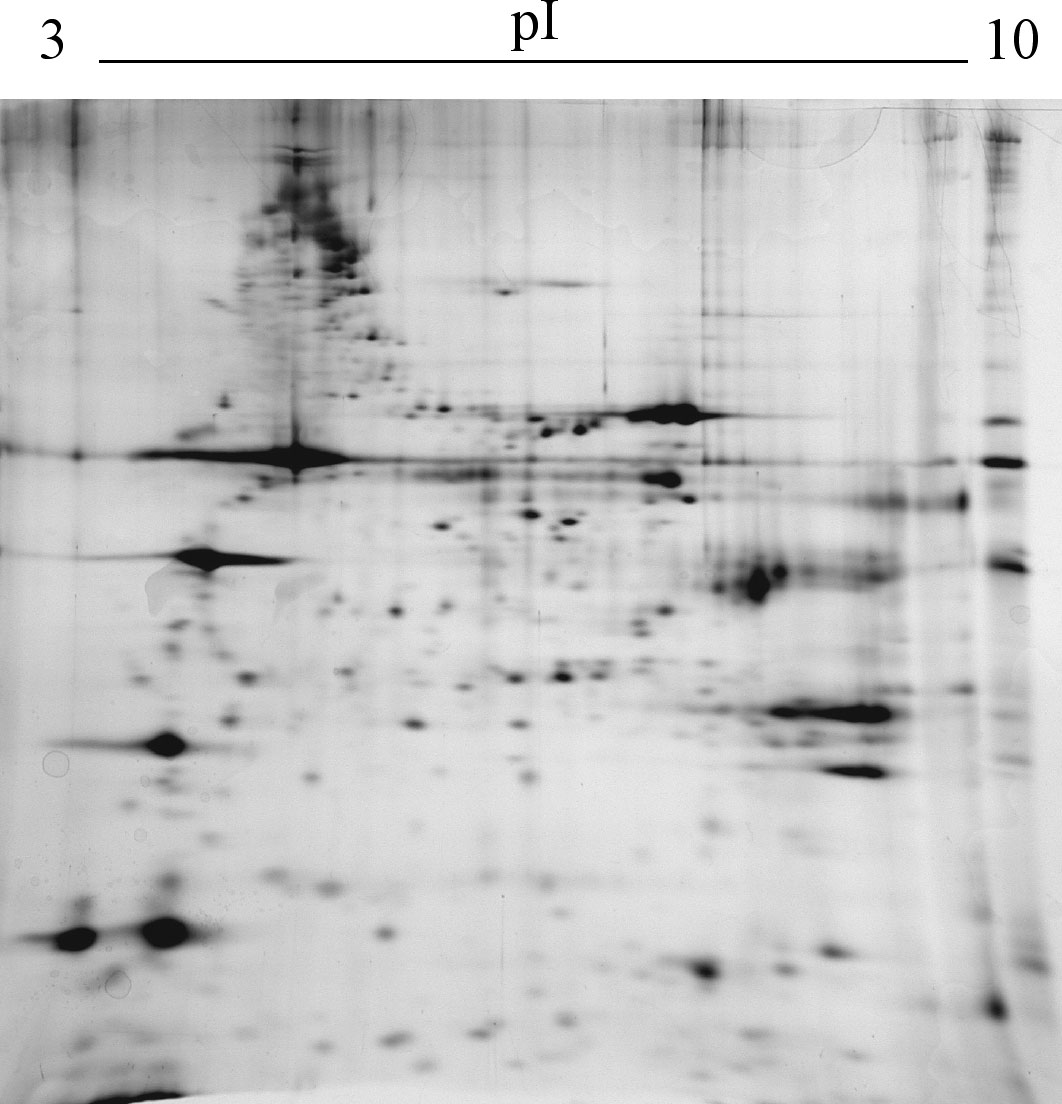
Research activity are performed on animal models using physiological, biochemical and molecular approaches, such as tissue and cell isolation and fractionation (BBMV and BLM vesicles, cell suspensions); enzymatic measurements; radioactive tracers; fluorescent dyes for ions (SPQ), membrane potential (DiS-C2(5)) and pH (AO and BCECF); kinetic analysis of membrane transport proteins; ELISA; EIA; immunohistochemistry; DNA and RNA isolation, PCR, RT-PCR, SCGE, bioinformatics, data bank consulting, computer assisted sperm analizer (CASA), automated sperm morphometry analysis (ASMA), proteomic analysis (2D-electrophoresis, bioinformatic analysis).
Relevant publications
1. Zilli L., Schiavone R., Zonno V., Storelli C., Vilella S, 2003. Evaluation of DNA damage in Dicentrarchus labrax sperm following cryopreservation. Cryobiology; 47:227-235.
2. Zilli L., Schiavone R., Zonno V., Storelli C., Vilella S., 2004. ATP concentration and b-D-glucuronidase activity as indicators of sea bass semen quality. Biology of Reproduction 70:1679-1684.
3. Zilli L., Schiavone R., Zonno V., Rossano R., Storelli C., Vilella S., 2005. Effect of cryopreservation on sea bass sperm proteins. Biology of reproduction 72:1262-1267.
4. Schiavone R., Zilli L., Vilella S., Fauvel C., 2006. Human chorionic gonadotropin induces spermatogenesis and spermiation in 1-year-old European sea bass (Dicentrarchus labrax): Assessment of sperm quality. Aquaculture 255:522-531.
5. Schiavone R., Zilli L., Storelli C., Vilella S., 2008. Identification by proteome analysis of muscle proteins in sea bream (Sparus aurata). European Food Research and Technology 227: 1403-1410.
6. Zilli L., Schiavone R., Storelli C., Vilella S., 2008. Molecular mechanisms determining sperm motility initiation in two sparids (Sparus aurata and Lithognathus mormyrus). Biology of reproduction 79: 356-366.
7. Zilli L., Schiavone R., Storelli C., Vilella S., 2008. Effect of cryopreservation on phosphorylation state of proteins involved in sperm motility initiation in sea bream. Cryobiology 57: 150-155.
8. Zilli L., Schiavone R., Chauvigné F., Cerdà J., Storelli C., Vilella S., 2009. Evidence for the involvement of aquaporins in sperm motility activation of the teleost gilthead sea bream (Sparus aurata). Biology of reproduction, 81: 880-888.
9. Zilli L., Beirão J., Schiavone R., Herraez M.P., Cabrita E., Storelli C., Vilella S, 2011. Aquaporin inhibition changes protein phosphorylation pattern following sperm motility activation in fish. Theriogenology, 76:737-44.
10. L Zilli, R Schiavone, C Storelli, S Vilella 2012. Molecular mechanism regulating axoneme activation in marine fish: a review. International Aquatic Research 4 (1), 1-11.
11. Schiavone R., Zilli L., Vilella S., 2012. Changes in hormonal profile, gonads and sperm quality of Argyrosomus regius (Pisces, Scianidae) during the first sexual differentiation and maturation. Theriogenology,77:888-898.
12. Beirão J., Zilli L., Vilella S., Cabrita E., Schiavone R., Herráez M. P, 2012. Improving Sperm Cryopreservation with Antifreeze Proteins: Effect on Gilthead Seabream (Sparus aurata) Plasma Membrane Lipids. Biology of Reproduction, 86 (2) 59, 1-9.
13. Beirão J., Zilli L., Vilella S., Cabrita E., Fernández-Díez C., Schiavone R., Herráez M.P. 2012. Fatty acid composition of the head membrane and flagella affects Sparus aurata sperm quality. Journal of Applied Ichtiology, 28:1017-1019.
14. F Chauvigné, M Boj, S Vilella, RN Finn, J Cerdà. 2013. Subcellular Localization of Selectively Permeable Aquaporins in the Male Germ Line of a Marine Teleost Reveals Spatial Redistribution in Activated Spermatozoa. Biology of Reproduction (2013) 89(2):37, 1–17.
Group of Biology of reproduction and Developmental Biology
Department of Life and Environmental Sciences
Polytechnic University of Marche
Contact
Dr. Oliana Carnevali
o.carnevali@univpm.it
Phone: +39 0712204990
Address
Dipartimento di Scienze della Vita e dell’Ambiente
Università Politecnica delle Marche
Via Brecce Bianche
60131 Ancona
Group description
The Department of Life and Environmental Sciences (DISVA), includes more 100 scientists and technicians organized in 20 interdisciplinary laboratories, spanning from the analytical and organic chemistry to the biophysics, from the cellular to the molecular biology, biochemistry and genetics, from the microbiology and biotechnology to algal, plant and animal taxonomy, physiology and reproduction, from the marine biology and eco-toxicology to oceanography and paleo-ecology, from the geology and risk prevention to civil protection. These laboratories allow covering a wide spectrum of scientific investigations and applied research and to represent a centre of excellence in the field of life, environmental research, with special focus on marine sciences. In the last 5 years researchers of DISVA published approximately 200 papers per year on International Journals with an average of 2500 citation/year.
The scientists working at Department collaborate with several national and international research centres, and contribute to Large Scale European Facilities. Direct collaboration and agreements have been established with a number of Universities and research institutions on a global scale (from South America to US and Canada, from Australia and New Zealand to Madagascar, all European countries, Israel and Japan, from Russian to Vietnam) and DISVA labs have been established in Vietnam and Indonesia within the frame of bilateral collaborations. The researchers of the DISVA are directly involved and/or coordinate several international research projects (including EU programs from FP5 to FP7) and networks of excellence as well as several national strategic research projects. DISVA’s personnel participate to the National Research Antarctic Program and is involved in International Expeditions to Arctic.
The DISVA offers graduate and post-graduate degree courses in Biological Sciences subdivided into two 3-years courses on general preparatory subjects and three 2-years postgraduate courses of specialisation:
First Level Degree Courses (three years)
• Biological Sciences
• Environmental Control Science and Civil Protection
Postgraduate Degree (Master) Courses (two years)
• Applied Biology
• Marine Biology
• Environmental Sustainability and Civil Protection
School of Doctorate in Science (three years) PhD courses on the following Curricula:
• Marine biology and ecology
• Bio-molecular sciences
• Environmental and civil Protection
The DISVA is the Headquarter of International Masters on “Tropical Marine Biodiversity and Bioactive Compounds” and “Management of Emergencies and Natural Disasters” and host the first Master Online in Marine Biology (on the FUNIBER platform).
Main lines of research
Gamete quality in teleost By molecular, cellular and morphological tools can be evaluated the quality of gametes. These results may be used as early warning signal to avoid the collapse of the Ichthyic resources and for a better management of natural resources.
Reproductive endocrinology and physiology Study in new species for aquaculture including tropical fish reproduction for aquarium trade and for restocking of endangered species in natural habitat
Development of new biotechnologies for environment-friendly aquaculture. USE of Natural products with immuno-modulator activity properties, probiotics, prebiotics, will be identified by biological tests to develop new commercial feed additives and to optimize the administration protocols to improve animal welfare ( improvement of stress tolerance, growth food intake and health status)
Reproductive toxicology to monitor the environmental risk. One of the goals is the development of biomarkers to determine how contaminants alter the health of wildlife populations. To evaluate if remedial action (i.e. removal and/or treatment of dredged materials) reduces toxic effects of the exposure to mixtures of contaminants (i.e. water column, dredged materials), a wide spectrum of aspects as reproduction, endocrinological axis, immune system and genotoxic aspects, on different marine fish models (Sparus aurata, Solea solea, Fundulus heteroclitus, Danio rerio) by genomic and proteomic approach.
Links Department of Life and Environmental Sciences (DISVA): www.disva.univpm.it
Master on line in Marine Biology: wwwfuniber.it
Relevant publications
1. MARADONNA F, GIOACCHINI G.,FALCINELLI, S, BERTOTTO B, RADAELLIG., IKE OLIVOTTO1, OLIANA CARNEVALI (2013) Probiotic Supplementation Promotes Calcification in Danio rerio Larvae: A Molecular Study PlosOne | Volume 8 | Issue 12 | e83155
2. CARNEVALI O., AVELLA M.A., GIOACCHINI G. (2013). Effects of probiotic administration on zebrafish development and reproduction Gen Comp Endocrinol. 188:297-302
3. BENATO F, SKOBO T, GIOACCHINI G, MORO I,CICCOSANTI F, PIACENTINI M, FIMIA GM, CARNEVALI O AND LUISA DALLA VALLE (2013). Ambra1 knockdown in zebrafish leads to incomplete development due to severe defects in organogenesis, Autophagy Volume 9, Issue 4, 476 – 495
4. MARADONNA F., EVANGELISTI M.,GIOACCHINI G, MIGLIARINI B.,OLIVOTTO I., AND CARNEVALI O (2013) Assay of vtg, ERs and PPARs as endpoint for the rapid in vitro screening of the harmful effect of Di-(2-ethylhexyl)-phthalate (DEHP) and phthalic acid (PA) in zebrafish primary hepatocyte cultures . Toxicology in Vitro, Volume 27, Issue 1, , Pages 84–91
5. AVELLA MA, PLACE A., DU SJ, WILLIAMS E, SILVI S, ZOHAR Y, CARNEVALI O (2012)Lactobacillus rhamnosus Accelerates Zebrafish Backbone Calcification and Gonadal Differentiation through Effects on the GnRH and IGF Systems PLoS ONE 7(9): e45572. doi:10.1371/journal.pone.0045572
6. LOMBARDO F, GIORGINI E, GIOACCHINI G, MARADONNA F, FERRARIS P, CARNEVALI O. (2012)Melatonin effects on Fundulus heteroclitus reproduction. Reproduction, Fertility and Development, http://dx.doi.org/10.1071/RD11267
7. RIBECCO C., HARDIMAN G., SASIK R, VITTORI S, CARNEVALI O. (2012) Teleost fish (Solea solea): A novel model for ecotoxicological assay of contaminated sediments. Aquatic Toxicology 109:133-142.
8. GIOACCHINI, G, GIORGINI, E,. MERRIIELD D L, HARDIMAN G, BORINI A, VACCARI, L; CARNEVALI, O (2012) Probiotics can induce follicle maturational competence: the Danio rerio case. Biology of Reproduction. published ahead of print November 16, 2011, doi:10.1095/biolreprod.111.094243
9. RIBECCO C., BAKER M. E., SASIK R, ZUO Y, HARDIMAN G., CARNEVALI O. (2011)Biological effects of marine contaminated sediments on Sparus aurata juveniles AQUATIC TOXICOLOGY 104 ( 3-4)308-316 DOI: 10.1016/j.aquatox.2011.05.005
10. CARNEVALI O, GIOACCHINI G, MARADONNA F, OLIVOTTO I AND MIGLIARINI B. (2011) Melatonin induces follicle maturation in Danio rerio. PLoS ONE 6(5): e19978.doi:10.1371/journal.pone.0019978
11. AVELLA M.A., OLIVOTTO I., SILVI S., RIBECCO C., CRESCI A., PALERMO F., POLZONETTI A., CARNEVALI O(2011). Use of Enterococcus faecium to improve common sole (Solea solea ) larviculture. Aquaculture 31, 384–393
12. MIGLIARINI B., PICCINETTI C.C. , MARTELLA A., MARADONNA F. , GIOACCHINI G., CARNEVALI O.(2011). Perspectives on endocrine disruptor effects on metabolic sensors. Gen. Comp. Endocrinol. 170, 416–423.
13. GIORGINI E., CONTI C., FERRARIS P., SABBATINI S., TOSI G., RUBINI C.,VACCARI L., GIOACCHINI G., CARNEVALI O. (2010). Effects of Lactobacillus rhamnosus on zebrafish oocyte maturation: an FTIR imaging and biochemical analysis. Anal. Bioanal. Chem 398(7-8), 3063-3072.
14. GIOACCHINI G., MARADONNA F., LOMBARDO F., BIZZARO D., OLIVOTTO I., CARNEVALI O. (2010) Increase of fecundity by Probiotic administration in zebrafish (Danio rerio). Reproduction 140, 953–959.
15. CARNEVALI O., TOSTI L , SPECIALE C, PENG C, ZHU Y., MARADONNA F. (2010) DEHP impairs zebrafish reproduction by affecting critical factors in oogenesis PLOS one. 5 (4),1-6.